Following article is first in the series of articles on the Indian Pharmaceutical Industry, the first article is written to familiarize ourselves with the terminology or the jargon’s of the pharmaceutical industry. We will briefly touch upon terms like API, Intermediates, Formulations, Innovator drug, Generic drugs, life cycle development etc.
The Indian Pharmaceutical industry is about $ 17 bn industry (2016) with as many as 20,000 registered companies (includes MNC’s and small scale units) directly or indirectly involved in the business of selling medicines. India has the distinction of being the lowest cost producer of medicine in the world. India also has the feather of being the largest exporter of generic drugs in the world, we have some great franchises like Lupin, Sun Pharma etc.
Terminologies
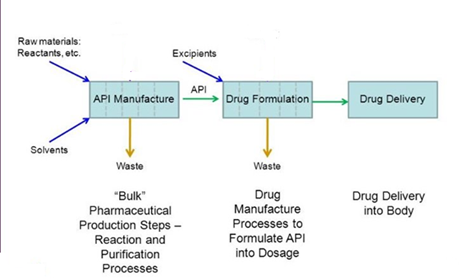
API – Active Pharmaceutical Ingredient –
It is the basic drug itself with the desired medicinal pharmaceutical properties, also known as bulk drug.
Intermediates –
Most chemical reaction are step wise, that is they take more than one elementary step to complete and the intermediary formed in the process of making an API is called an intermediate.
Finished Dosage or Formulation –
It is the form in which the drug is consumed by us. A dosage form of a drug is usually composed of two things: The API, which is the drug itself; and an excipient, which is the substance of the tablet, or the liquid the API is suspended in.
Pharma Supply Chain from Intermediates to API maker to Formulations maker.
Oncology –
Oncology deals with the prevention, diagnosis, and treatment of cancer.
Tentative Approval –
Tentative Approval is granted prior to patent exclusivity expiry for a blockbuster drug, however companies getting tentative approval cannot market the drugs in USA until they receive final approval.
Blockbuster Drug –
A blockbuster drug is an extremely popular drug that generates annual sales of at least $1 billion for the company that sells it. Blockbuster drugs are commonly used to treat common medical problems like high cholesterol, diabetes, high blood pressure, asthma and cancer.
DMF- Drug Master File –
API manufacturers need to file a document known as Drug master File (DMF) with regulatory bodies. A Drug Master File (DMF) is a submission to the FDA that may be used to provide confidential detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of one or more human drugs.
New Drug Application –
The final step formally taken by a drug sponsor, wherein it applies to the Food and Drug Administration (FDA) for the approval required to market a new drug in theU.S. An NDA is a comprehensive document with 15 sections that includes data and analyses on animal and human studies, the drug’s pharmacology, toxicology and dosage, and the process to manufacture it. When an NDA is submitted, the FDA has 60 days to decide whether to file it for review, or reject it because some required information is missing. The goal of the FDA’s Center for Drug Evaluation and Research (CDER) is to review and act on at least 90% of NDAs for standard drugs within 10 months after the applications are received, and six months for priority drugs.
ANDA (Abbreviated New Drug Application) –
Abbreviated New Drug Applications are “abbreviated” since they do not require the applicant to conduct clinical trials and require less information than a New Drug Application. If an ANDA is approved, the generic drug will be listed in the Orange Book, which lists all medicines the FDA has found to be safe and effective. An ANDA contains all the information the it needs to evaluate on how safe and effective a proposed generic drug is compared with its brand-name equivalent. The FDA will not approve the generic unless it is equally safe and effective.
Bio Similars –
Bio similar is an approved drug that it is highly similar to an FDA-approved biologic product, and has no clinically meaningful difference in safety or effectiveness from the originally approved product. However, bio similar is not chemically identical to the drug they refer, and may include slight differences. Medical practitioners or pharmacists don’t have the liberty to give a bio similar drug in place of the biologic.
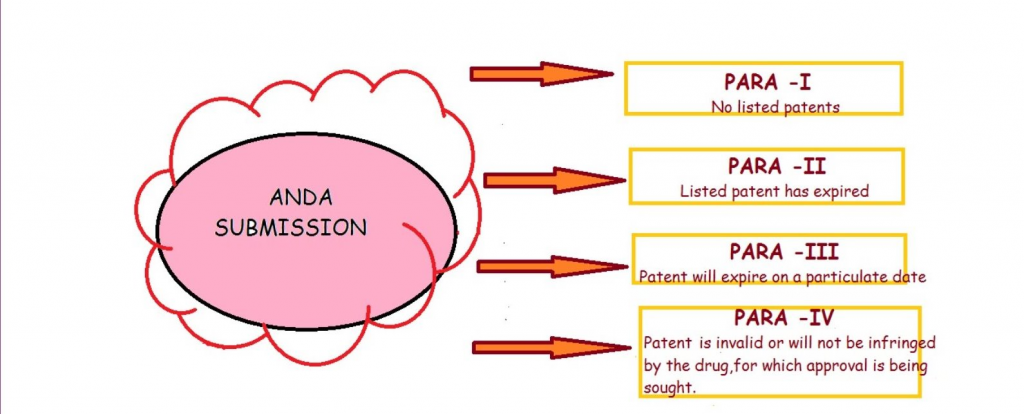
PARA 1 –
A Para 1 filing is made during the launch of a generic drug when the innovator has not provided the required information in the orange book.
PARA 2 –
Para 2 filing is made when the drug is already off patent.
PARA 3 –
Para 3 filing is made when the applicant does not have any plans to sell the generic drug until the original drug is off patent.
PARA 4 –
A Para IV filing for the launch of generic drug is made when the applicant believes its product or the use of its product does not infringe on the innovator patents or where the applicant believes such patents are not valid or enforceable.
Acute Disease –
An acute disease is a disease with a rapid onset and/or a short course.
Chronic Disease –
A chronic condition is a human health condition or disease that is persistent or otherwise long-lasting in its effects.The term chronic is usually applied when the course of the disease lasts for more than three months.
CRAMS – Contract Research and Manufacturing –
One of the fastest growing segments in the pharmaceutical and biotechnology industry. It pertains to outsourcing research services/ manufacturing products to low-cost providers with world class standards.
Generic Drug –
A generic drug is a drug that is not branded but is similar to a branded or reference listed drug in terms of dosage, administration and performance.
505 (b)(2) –
The 505(b)(2) new drug application (NDA) is one of three U.S. Food and Drug Administration (FDA) drug approval pathways and represents an appealing regulatory strategy for many clients.A 505(b)(2) NDA contains full safety and effectiveness reports but allows at least some of the information required for NDA approval, such as safety and efficacy information on the active ingredient, to come from studies not conducted by or for the applicant.
Biologics –
A biologic is manufactured in a living system such as a microorganism, or plant or animal cells. Most biologics are very large, complex molecules or mixtures of molecules. Many biologics are produced using recombinant DNA technology.
Exclusivity Period –
Exclusivity period refers to certain delays and prohibitions on approval of competitor drugs available under the statute that attach upon approval of a drug or of certain supplements. Exclusivity period was designed to promote a balance between new drug innovation and greater public access to drugs that result from generic drug competition.
Orange Book –
The publication Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the Orange Book) identifies drug products approved on the basis of safety and effectiveness by the Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act (the Act) and related patent and exclusivity information.
Purple Book –
The “Purple Book” lists biological products, including any biosimilar and interchangeable biological products, licensed by FDA under the Public Health Service Act (the PHS Act). The Purple Book includes the date a biological product was licensed under 351(a) of the PHS Act and whether FDA evaluated the biological product for reference product exclusivity under section 351(k)(7) of the PHS Act.
CCS (Custom Chemical Synthesis) –
Custom Chemical synthesis is a purposeful execution of chemical reactions to obtain a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions. In modern laboratory usage, this tends to imply that the process is reproducible, reliable, and established to work in multiple laboratories.
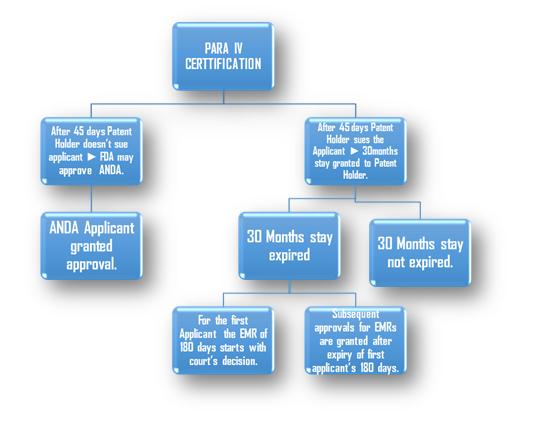
FTF (First to File) –
First to file is another category where even before the first five years are over a company can challenge the drug if approved that company gets an 180 days exclusive approval to market its generic version of the Innovator drug. This can prove very lucrative for the challenger if granted. On the other hand there are Litigation Risks where the Innovator tries to prove that the challenger has infringed on its patent/process while developing the generic version.
Innovator Drugs –
A generic drug is bioequivalent to a drug that has a brand name, also called an innovator drug. It will have a different name and will look different from its innovator counterpart, but the active ingredients will be the same.
Dispensing –
Selling out properly on a lawful prescription. A prescription can only be filled by a registered pharmacist, veterinarian, dentist or member of the medical profession. The law requires that a prescription be written only for patients that are under doctor’s care.
CFA (Clearing and forwarding agents) –
These organizations are primarily responsible for maintaining storage (stock) of the company’s products and forwarding SKUs to the stockist on request. Most companies keep 1–3 CFAs in each Indian state. On an average, a company may work with a total of 25–35 CFAs. The CFAs are paid by the company yearly, once or twice, on a basis of the percentage of total turnover of products.
Stockist –
He is the distributor, who can simultaneously handle more than one company (usually, 5–15 depending on the city area), and may go up to even 30–50 different. They pay for the products directly in the name of the pharmaceutical company after 30 to 45 days.
Bio-equivalence –
Bio-equivalence is the similarity between two drugs which essentially means that they both have the same effect on the patient. Bio equivalence means that two drugs release their active ingredient into the bloodstream in the same amounts and at the same rate. When assessing how well a generic drug works, scientists evaluate its bio equivalence to the name-brand version.
CGMP –
Current Good Manufacturing Practice, CGMP is to follow the current guidelines to produce the best quality pharmaceutical products.
Pediatric Exclusivity –
6 more months added to existing patent exclusivity.
Orphan Drug Exclusivity –
To treat a disease that affects fewer than 200,000 people in the United States.The orphan drug law offers tax breaks and a seven-year monopoly on drug sales to induce companies to undertake the development and manufacturing of such drugs, which otherwise might not be profitable because of the small potential market.
Para-Filings
Drug Types
There are two types of drug:
- Innovator drug
- Generic drug
Manufacturing stages of a medicine.
-
Innovator drug
Drug is build up from the scratch, thus a much rigorous process and even the patent is filled which leads into huge prices of drug in its patent life as there is no competitor. And when a drug goes off patent at that time price crashes roughly 70%. Usually the player who gets the 180 days exclusivity corners 60% of this market as his brand gets established.
Drug Development Life Cycle
Drug Development Life Cycle is a complex and a very long process which lasts 10-15 years. 1000 of tests are conducted on the drug across the country. Research teams from various labs work hard day and night to analyze the disease. When any new drug is launched there are clinical trials done with first to file. We have drug development phases which are regulated by the authority like FDA, these phases are phase 1, phase 2, phase 3 etc.
The video embedded will explain the whole process of Drug Discover.
Discovery
Represents first stage. It is the process by which drugs are discovered and/or designed. We identify cellular and genetic factors that play a role in specific diseases and it can take 10-15 years for drug approval.
Development
It is a phase where promising compound is transformed into a marketable product. It is a process of taking a new chemical through various stages necessary to allow it to be tested in human clinical trials.
Pre-Clinical Tests
The beginning of the drug approval process. To see the potential effects on humans, tests are performed on: Isolated tissues, Cell Cultures and Animals. Company decides whether to put the drug into the human testing process, based on the marketability of the product and their financial situation. On average, only one compound in a thousand will actually make it to human testing
IND Filings
The goal is to provide pre-clinical data of high quality to justify the testing of the drug on humans. FDA has 30 days to review the Investigational New Drug (IND) application. It must be filed annually until the completion of clinical testing. During this time patents are applied; patents last generally for 20 years. About 85% of all IND applications move on to begin clinical trials. If they succeed, there is a 20% chance of the product making it to the market.
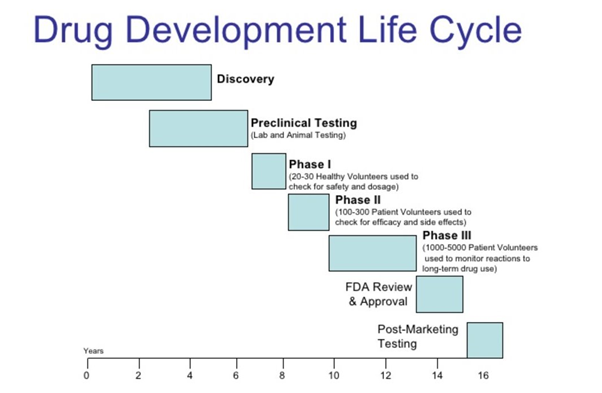
NDA Filing
Upon desirable results from Phase III, New Drug Application (NDA) will be submitted. NDA contains data supporting the efficacy and safety of the drug. Approval can take 2 month to several years, but on average, it takes around 18 to 24 months. Drugs are subject to ongoing review, making sure no adverse side effects appear. After FDA’s approval, the drug can be marketed and distributed.
Patent
Generally lasts for 20 years. Since most companies file for patent during pre-clinical trials, usually the patent is only good for another 10 years or so after it gains FDA approval. What can be patented – product, method and use.
2. Generic Drug
A generic drug is a medication created to be the same as an already marketed brand name drug in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use. These similarities help to demonstrate bio equivalence which means that a generic medicine works in the same way and provides the same clinical benefits as its brand name version. In other words, it can be treated as an equal substitute for its brand name counterpart.
In the next article i.e. part 2 of the series, we will take a look at other aspects of the indian pharma industry.
Disclaimers :
The information herein is used as per the available sources of bseindia.com, company’s annual reports & other public database sources. Alpha Invesco is not responsible for any discrepancy in the above mentioned data. Investors should seek advice of their independent financial advisor prior to taking any investment decision based on this report or for any necessary explanation of its contents
Future estimates mentioned herein are personal opinions & views of the author. For queries / grievances – support@alphainvesco.com or call our support desk at 020-65108952.
SEBI registration No : INA000003106
Readers are responsible for all outcomes arising of buying / selling of particular scrip / scrips mentioned here in. This report indicates opinion of the author & is not a recommendation to buy or sell securities. Alpha Invesco & its representatives do not have any vested interest in above mentioned securities at the time of this publication, and none of its directors, associates have any positions / financial interest in the securities mentioned above.
Alpha Invesco, or it’s associates are not paid or compensated at any point of time, or in last 12 months by any way from the companies mentioned in the report.
Alpha Invesco & it’s representatives do not have more than 1% of the company’s total shareholding. Company ownership of the stock : No, Served as a director / employee of the mentioned companies in the report : No. Any material conflict of interest at the time of publishing the report : No.
The views expressed in this post accurately reflect the authors personal views about any and all of the subject securities or issuers; and no part of the compensations, if any was, is or will be, directly or indirectly, related to the specific recommendation or views expressed in the report.
Stay Updated With Our Market Insights.
Our Weekly Newsletter Keeps You Updated On Sectors & Stocks That Our Research Desk Is Currently Reading & Common Sense Approach That Works In Real Investment World.